Phage-Biosensors.
At Aqsens Health we focus on developing screening tests for diseases that are hard to diagnose, and where the benefit of an early diagnosis is the biggest. At the core of our technology are engineered phage-biosensors, whose high specificity and adaptability make them unmatched in disease and pathogen detection.
What are phages?
Phages, or bacteriophages, are widely present in nature: they are the most numerous biological entities on earth, outnumbering all other organisms. Some estimate that the number of phage particles on Earth is around a trillion phages for every grain of sand in the world.
Phages are viruses that specialize in infecting only bacterial cells, leaving mammalian cells unharmed. Phages can resist harsh conditions from extreme heat to extreme coldness, and thrive wherever bacteria live.
In the human body billions of different phages play an important role in our well-being. They protect us against pathogens, modulate the composition of our gut microbiome, and impact our bacterial balance, immune function and overall health.
The ability of phages to thrive in various environments demonstrates the strength of natural selection. This adaptability makes them a unique basis for developing biosensors that can identify difficult-to-detect targets. Aqsens Health’s biosensor utilizes this natural ability to rapidly evolve and to detect different diseases and conditions from cancer to pathogen infections like malaria.
Phage research has achieved several Nobel prizes:
Viral Replication Mechanism – 1969
DNA Repair Mechanism – 2015
Phage Display – 2018
CRISPR Gene Editing – 2020
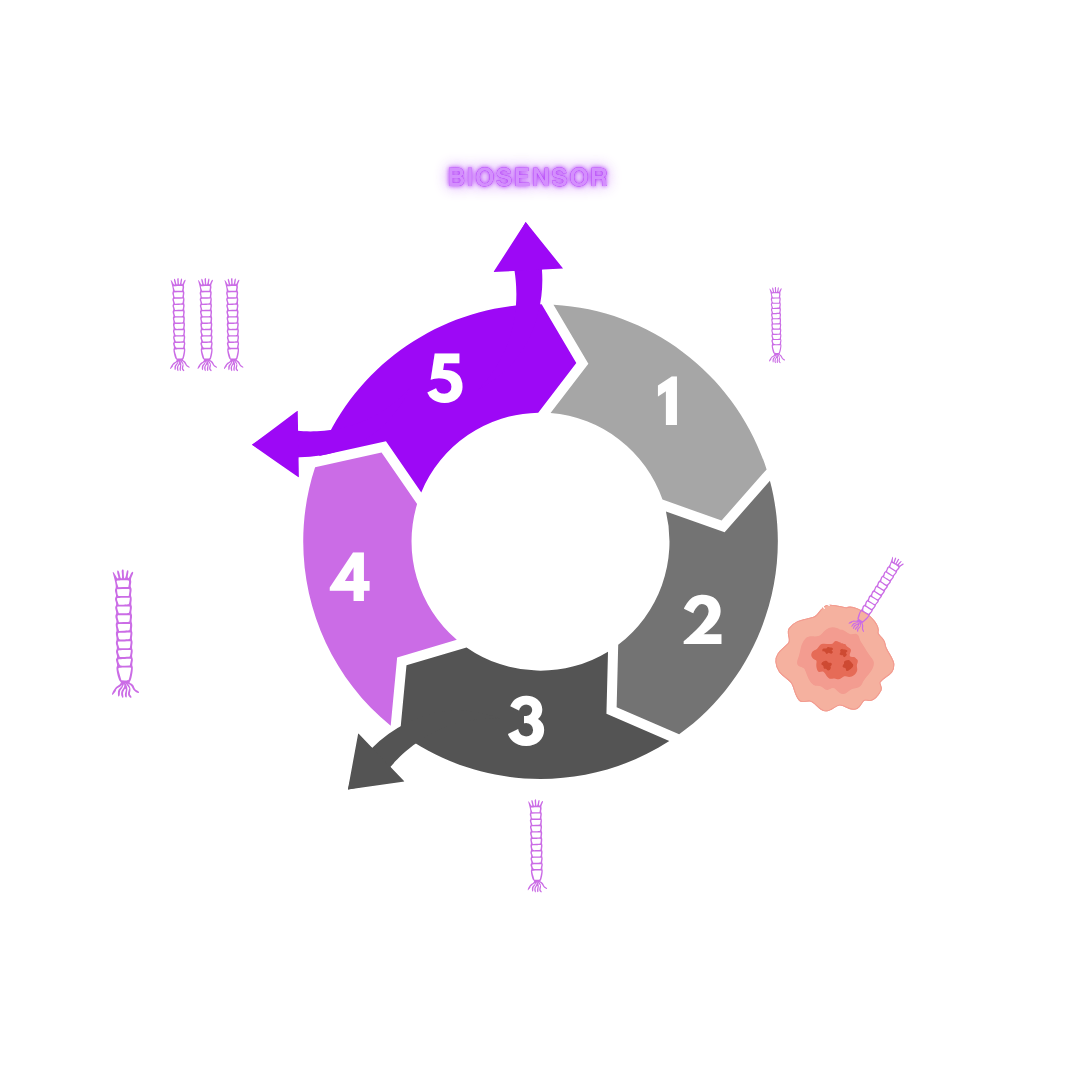
Biosensor development
The biosensor development starts from selecting specific phage strains, which are then gradually evolved and engineered to be able to recognize disease-specific biomarkers in the human immune system.
Engineering specific phage peptides enables not only fine-tuning of affinity to target biomarkers, but also optimization of signal strength through adjustable reporter-dye interactions. This programming is achieved through a proprietary biopanning process.
When phages bind to specific biomarkers for a particular disease and interact with the dye-reporter, the color intensity of the sample changes accordingly. These changes in color intensity can be measured using two methods: a standard absorbance reader or a smartphone equipped with the AQ MOBI platform and its application software.
How can phage-biosensors compliment existing diagnostic methods?
-
Phages have several advantages that can complement antibody-based methods, including longer shelf life and increased stability. The stability of phages is attributed to their unique structure and composition. They are highly resistant to environmental factors such as temperature and pH changes, allowing them to remain viable for extended periods of time. The long shelf life and increased stability of phages make them ideal for applications in diagnostics.
-
Phage-biosensors are as specific as other current detection methods. This specificity is due to the ability of phages to recognize and bind to specific target molecules, such as proteins, carbohydrates, and nucleic acids. By engineering phages to bind to very specific target molecules, Aqsens Health can create biosensors that are highly sensitive and specific for the detection of those molecules.
-
Phage-biosensors are extremely cost-efficient, making them well applicable alongside other conventionally used detection methods. The low cost of biosensors arises from their simplicity, low production costs, reduced instrumentation needs, high stability, and scalability. These advantages make phage-based biosensors attractive for various applications in healthcare and diagnostics.
-
Phage-based sensors can be developed significantly faster than other types of sensors. They can be grown in standard bacterial cultures, with the option of scaling up production using bioreactors for larger quantities. They offer straightforward purification steps and easy scalability to high-throughput systems, and batch production can be readily adapted to different phage types and target bacteria. Quality control is easy throughout the whole process.